Part 1 | Part 2 | Part 3 | Part 4 | Part 5 | Part 6 | Part 7 | Summary
Diffusion
If I take a drop of red food coloring and put it in a glass of water, what happens? The red food coloring begins spreading out until, eventually, the entire glass of water is a light pink color.
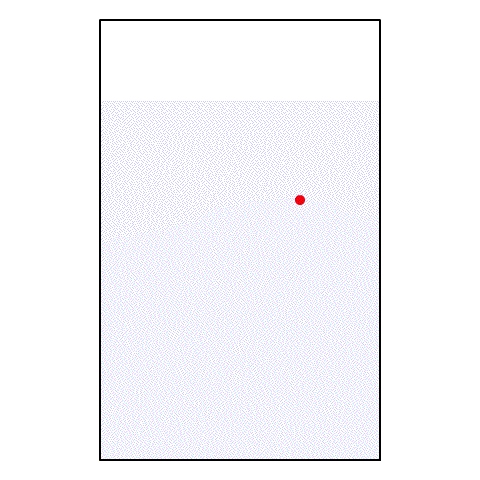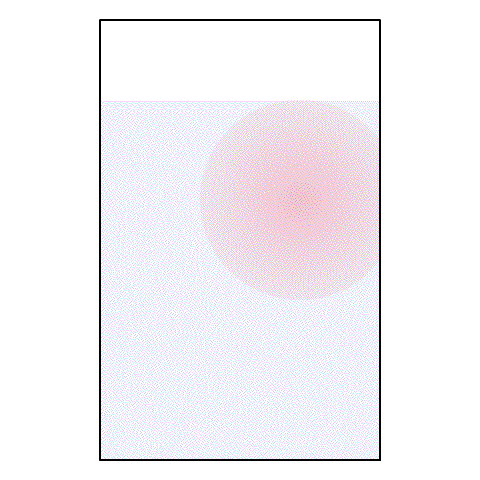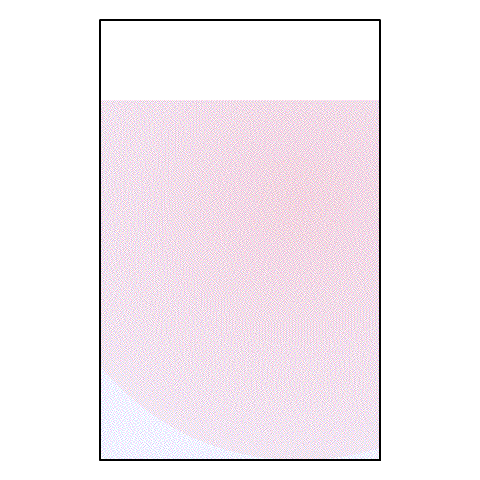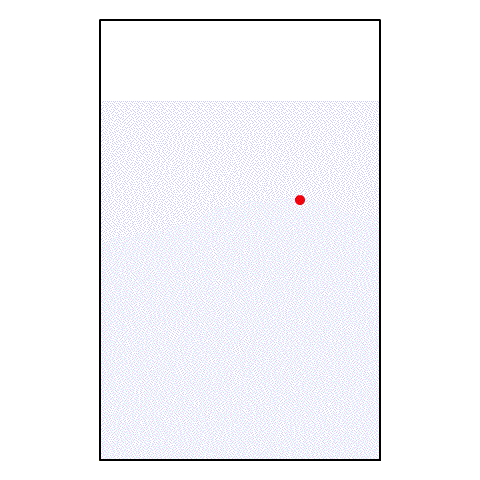 |
| A drop of red food coloring in a glass of water |
This process is known as diffusion. Diffusion is the flow of particles from regions of high concentration to regions of low concentration. What does this look like on a molecular scale?
 |
| An inaccurate and misleading visualization of diffusion on a molecular scale |
If the red dots represent particles of red dye, then we might think that diffusion looks something like this on a molecular scale. The red dye particles are moving away from each other, spreading out until they are evenly distributed. But how do the red dye particles know where to move? Can they detect regions of high concentration and low concentration? Even if particles could detect concentrations across distances, why would they want to move to a region where it is less crowded? Do particles have goals and desires?
For most of us, diffusion makes sense because we have lots of firsthand experience with it. We see diffusion happening in our everyday lives. It seems natural and intuitive. But few of us understand the local mechanisms that drive diffusion. We haven’t tried to drill down and ground our understanding in anything beyond the textbook definition of diffusion. This can result in misconceptions, such as the inaccurate and misleading visualization of diffusion on a molecular scale pictured above. Red dye particles don’t move away from each other; they move randomly. So how does diffusion happen? To figure that out, we will start with what we know.
Part 1 | Part 2 | Part 3 | Part 4 | Part 5 | Part 6 | Part 7 | Summary
I made a wikipedia animation + caption to try to illustrate this topic a few years ago -- see top of Fick's law article. It has room for improvement though. :-D
ReplyDeleteHi Steve. I included an animation in part two that was inspired by your animation. Hope you don’t mind!
DeleteHi Steve. Great animation! I like how you highlight how an individual solute molecule still moves randomly even though there is a steady net flow of molecules to the right. I’d be curious to hear what you think of this lesson as it unfolds.
ReplyDelete